Manufacturers, Are You Complying with State Required Drug Takeback Programs?
Written-by: Deneen Fumich, RPh
Did you know that as a manufacturer, there are 21 separate Manufacturer Funded Drug Takeback programs, also called Waste Stewardship programs, that are required by law? Did you also know that many of the programs do not require a manufacturer to physically distribute, sell or ship, into the jurisdictions?
Basically, if your product, no matter how it was distributed, enters a consumer’s home in the regulated jurisdictions, then you, as the manufacturer, are required to participate.
Did you know that if your product is identified and that your company is not participating there are enforcement actions? March 2022, Pharma Solutions wrote an overview article; “The Complexities and Application of Drug Takeback Programs” identifying that manufacturers/producers will bear the cost and burden to implement very complex programs.
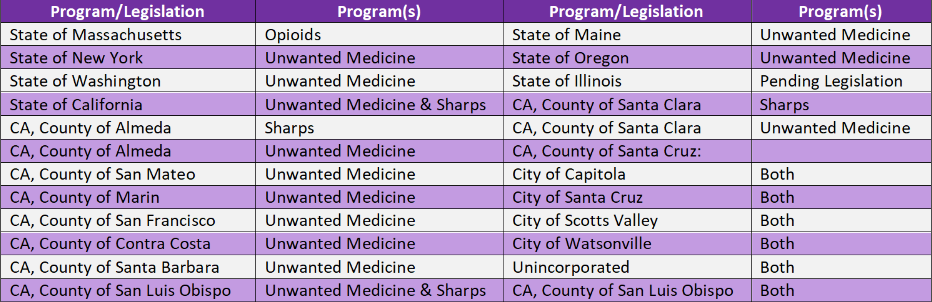
For your quick reference, below is the list of Program/Legislation.
For more information, there are 2 nationally recognized Drug Takeback compliance solutions:
Disclaimer: Pharma Solutions makes every effort to provide accurate information in the content it offers. However, the information provided is “as is” and Pharma Solutions makes no warranties, express or implied, as to the content provided. Pharma Solutions assumes no liability or responsibility for any errors or omissions in the content provided. Laws and regulations that are referenced herein may change over time and as such the content offered is not to be interpreted as advice. Pharma Solutions shall not be liable for any damages whatsoever incurred in connection with the use or performance of this information.




0 Comments