DSCSA Final Proposal Rules Submitted to OMB
Is 2022 the year for FDA to release Proposed Rules for the National Standards for the Licensure of Wholesale Drug Distributors and Third-Party Logistics Providers?
History November 27, 2013 saw the passage of Title II of the Drug Quality and Security Act, known as the “Drug Supply Chain Security Act (DSCSA)”. Within this Federal Act, the FDA and HHS was tasked to issue final regulations no later than two years after the enactment of the DSCSA (11/27/2015) for the National Standards for the Licensure of Wholesale Drug Distributors and Third Party Logistics Providers: Title II of the Drug Quality and Security Act | FDA.
Proposal
Finally, the long-delayed and awaited proposed rule from FDA has been submitted to the White House Office of Management and Budget, “OMB”, and is on the OMB’s agenda for 2022: Pending EO 12866 Regulatory Review (reginfo.gov). The regulations for the National Standards for the Licensure covers:
- Wholesale distributors minimum required information for licensing, qualifications, personnel, storage and handling, distribution records, violations and penalties
- Third Party Logistics Providers, “3PL” minimum requirements to be licensed by a state or requirements and process to be licensed by HHS.
Awaiting Approval
Until the federal rule is approved by OMB to be published by FDA, the impact to manufacturers, contract manufacturers, repackagers, contract repackagers, virtual manufacturers and wholesale distributors are unknown. As are the state changes unknown but will be monitored.
Monitoring Changes
From a State perspective, Pharma Solutions will be monitoring licensure changes for impacts to 3PL’s with attention to states identified by the International Warehouse Logistics Association’s meeting with OMB, View EO 12866 Meeting (reginfo.gov), are shaded red and green. 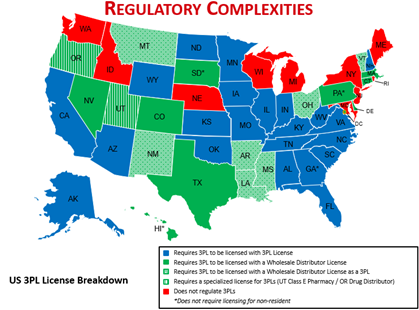




0 Comments