NABP DDA: New Process & “Ineligibility Matrix”
The National Association of Boards of Pharmacy® (NABP®) has posted on their website and has sent notices to current applicants just beginning their Drug Distributor and OTC Medical Device Distributor applications, information regarding the new streamlined process for the Drug Distributor Accreditation (DDA).
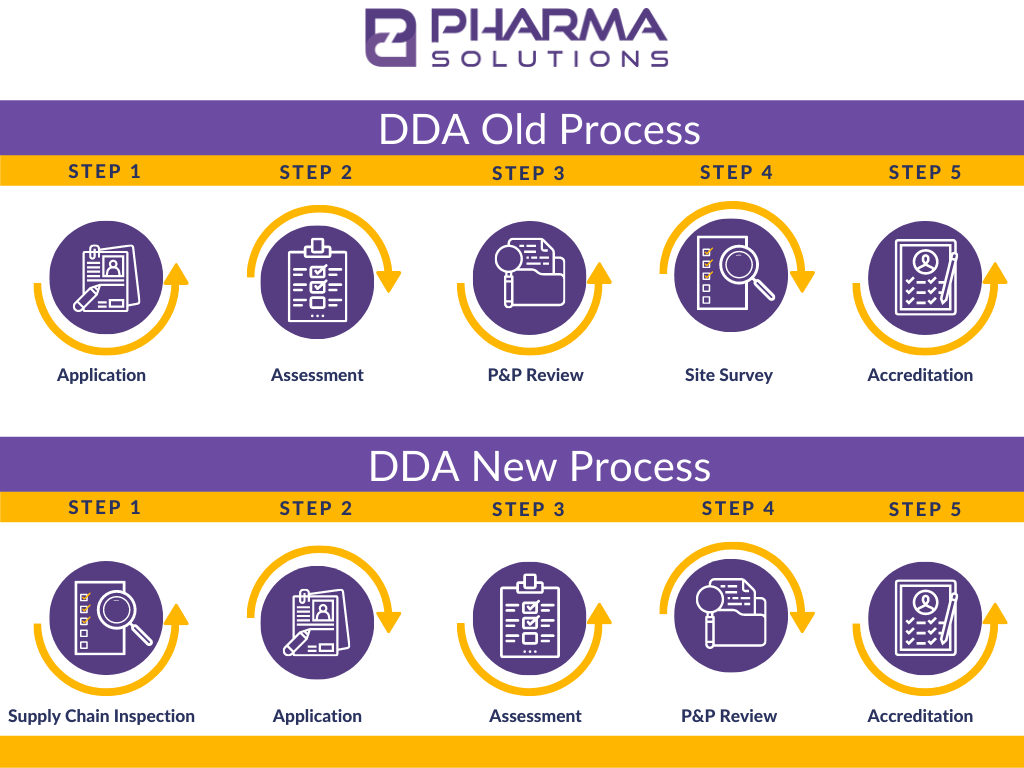
Background
Applicants currently in the pipeline for Drug Distributor Accreditation (DDA) can decide to either remain on the current path to complete the process or elect to begin following the new process guidelines. Effective January 2022, the streamlined requirements will apply to all distributor accreditation applicants. At that time, and going forward, the facility must first undergo and pass an onsite, unannounced Supply Chain Inspection to even be considered an applicant for accreditation.
Step 1: Supply Chain Inspection (formerly Site Survey)
The NABP’s process change to place the Supply Chain Inspection at the beginning of the accreditation process is a significant shift in the program that started over 15 years ago.
Eligibility Matrix
An Applicant’s first step should be to review NABP’s Distributor Accreditation Ineligibility Matrix (posted on their website) to determine whether they are “eligible” to apply for accreditation. There are significant categories that may now render a facility as “ineligible”(depending on the applicant business model) to even proceed to the second step of applying for a Supply Chain Inspection such as:
-
- Co-locating applicant and various other business models without adequate physical separation to secure drugs and devices and/or separate accounts to distinguish drug and device purchases made by different entities;
- Distributing for an unaccredited (NABP) wholesale distributor or not purchasing directly from and FDA registered manufacturer;
- Distributing for and/or sourcing from an unregistered FDA manufacturer;
- Distributing unapproved drugs and/or devices;
- Located in a private residence;
- Not storing controlled substances in accordance with DEA requirements;
- Operating in a facility not of suitable size and construction to facilitate cleaning, maintenance, and proper distribution operations;
- Unwilling to install and maintain a continuously operating electronic temperature/humidity recording and monitoring system;
- Unwilling to modify a business model that currently includes pharmacies in the supply chain;
- Unwilling to modify environmental controls to comply with regulatory and manufacturer label requirements (which may include having to install proper HAVC controls);
- Unwilling to obtain documented rationale for number and location of temperature/humidity probes (facility temperature mapping study);
Supply Chain Inspection
If after reviewing the ineligibility matrix the facility does not fall in to one of the listed categories and is still eligible, the second step is to apply for a Supply Chain Inspection (“SCI”) by submitting required documentation and fees via NABP’s online portal. After NABP has reviewed the documentation and applicant agrees to the new terms and conditions of the accreditation process, an onsite, unannounced inspection will be conducted by NABP at applicant’s facility within eight weeks. At that time, NABP will also contact applicant’s state Board of Pharmacy to let them know when the inspection will take place so that they may observe the inspection as well. Please note that a facility must be fully operational for at least thirty days before NABP will conduct an inspection, so SCI applications should not be submitted until facility can meet that condition before NABP would show up unannounced any time in that eight week period.
Inspection Results
After the SCI, NABP’s inspection report results will determine whether a facility is eligible or not to pursue accreditation by placing applicant in one of the three following categories:
- Eligible Expedited – means that NABP found very few/minimal observations and will fast track accreditation with it being awarded most probably within 14 days after applicant receives SCI report, given that applicant is actively working to resolve those few observations and that they continue to adhere to NABP criteria.
- Eligible – means that although NABP found observations, they were of the nature that they feel applicant can resolve them within NABP’s given time-period. Applicants will have thirty calendar (not business) days to respond to the SCI report with steps taken to correct any noncompliant items as well as any findings that applicant feels were made in error. No extensions will be granted.
- Ineligible – means that NABP found enough observations (and this is the inspector’s call) during the inspection that they will remove applicant from proceeding with the accreditation process. NOTE: At this point, an applicant would be free to immediately reapply for accreditation and go through the process again from the beginning; or resolve all observations that were found during the inspection and then reapply at that time; or choose to not pursue accreditation at all.
Within forty-five days of the SCI, NABP will provide applicant with a copy of the inspection report via email. The SCI report will also be shared with the applicant’s state Board of Pharmacy and/or other regulatory agency. NABP’s SCI report results are considered final at that time and applicant will have no right to appeal or contest the results. If an already accredited facility is found to be “ineligible” to continue with the reaccreditation process after inspection, NABP will immediately cancel their accreditation.
Step 2: Application
After the completion of the SCI, Applicants will need to submit an Application for DDA. The Application wholly changed in the last year as NABP began to request more information on each company, including photos, personnel information, and more.
Step 3: Application Review Report
After the Application materials are received and approved, applicants will be expected to go through the typical Application Review Report and SOP Review Report. The Application Review Report typically includes a review of application materials and ancillary information, including, but not limited to, the following:
- Ownership
- Business Model
- Customer List
- License List
- Vendor List
Step 4: SOP Review Report
The SOP Review Report is a straightforward review on how well a facility’s SOPs address NABP standards.
Additional Notes
Of note as well is that NABP’s terms and conditions do contain some new requirements that potential applicants should be aware of and fully understand as to binding requirements of the process. The NABP Drug Distributor Accreditation program is a must for pharmaceutical distributors due to regulatory and trade requirements Pharma Solutions can help your organization analyze gaps in policies and help you to develop, revise and/or supplement them, as well as implement the required controls and procedures in your operations to bring your activities up to NABP Drug Distributor Accreditation standards.




0 Comments