PBMs Turn Up the Pressure on Diabetic Test Strip Reimbursements
Two separate PBMs recently issued audits to an independent pharmacy because it was determined that the pharmacy was sourcing diverted test strips from an NABP DDA (fka VAWD®) Accredited facility.
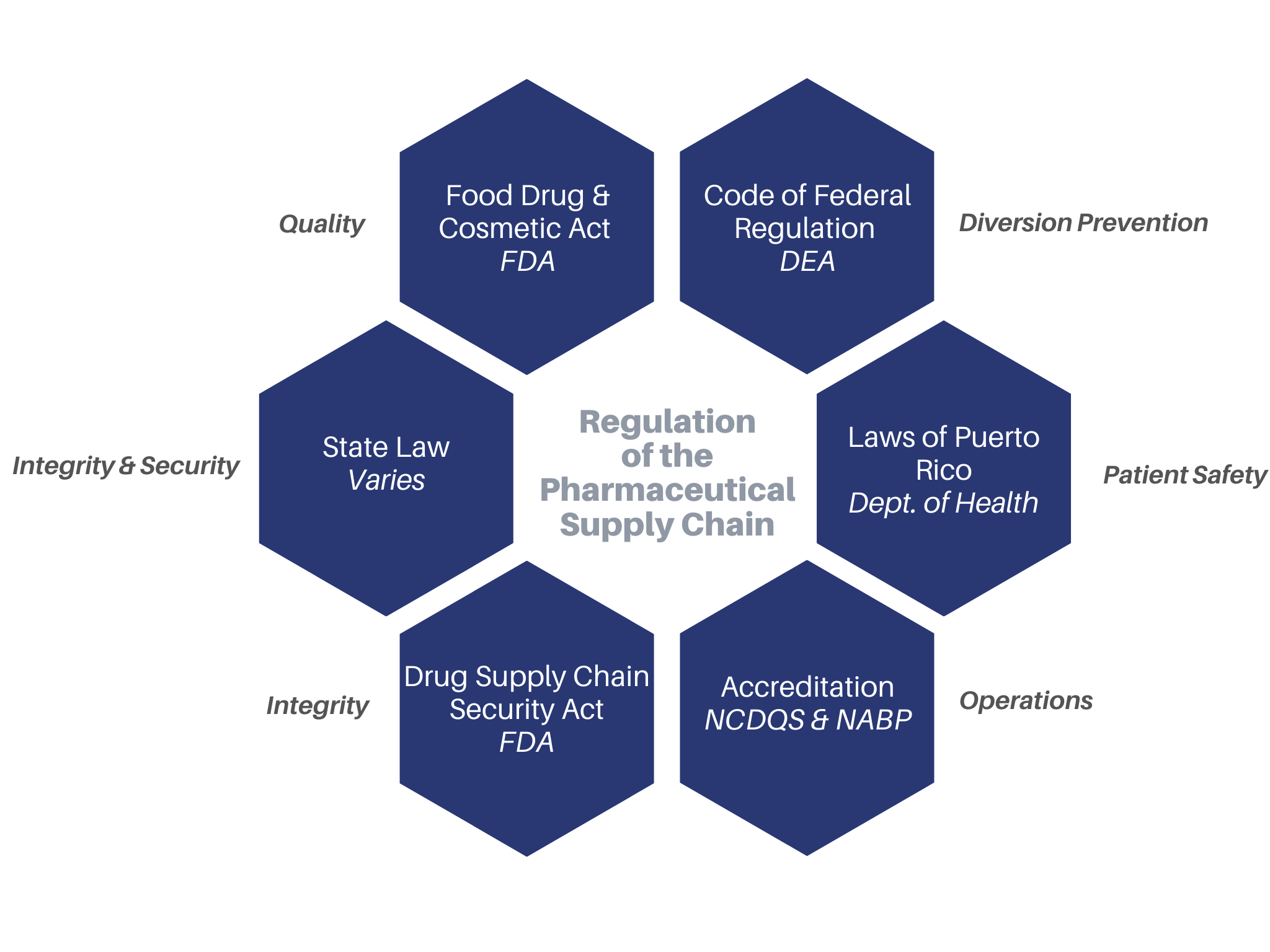
Against the backdrop of the controversial policy by Optum Rx which requires their pharmacy networks to source products only from NABP DDA (fka VAWD® Accreditation) facilities, at least three other PBMs have identified a key point of diversion: diabetic test strips. An Initial Discrepancy Evaluation Report from just one PBM on just test strips resulted in over $35,000 in “discrepancies” – or money to be reclaimed by the PBM.
In September 2016, the NABP created a separate Accreditation focused on diabetic testing supplies (OTC Medical Device Distributor Accreditation, fka VDIP® Accreditation). In January 2020, a Pharma Solutions client was awarded the very first Accreditation – over 3 years since the implementation of the program.
For more information on the Accreditation awarded as well as the program’s history, please read here
.
To ensure your company is operating in adherence to NABP DDA and industry best practices and mandated PBM practices, reach out to Pharma Solutions today.




0 Comments